312000796 - INTRODUCTION - EXHAUST EMISSION CONTROL SYSTEM
LAMBDA SENSOR
Specifications
Planar sensors are fitted upstream and downstream of the catalytic converter and inform the injection control unit of the progress of the combustion (stoichiometric ratio).To produce an optimum mixture the quantity of air drawn in by the engine must be equal to the theoretical quantity required to burn all of the fuel injected.In this case the Lambda factor ratio between the quantity of intake air and the theoretical quantity of air (required to burn all the fuel injected) is equal to 1.Therefore:
- Lambda = 1 ideal mixture
- Lambda > 1 lean mixture
- Lambda < 1 rich mixture
a. Rich mixture (not enough air)b. Lean mixture (too much air)The Lambda sensors are in contact with exhaust gases and generate an electrical signal with voltage dependent on oxygen level in the gas.This voltage alters abruptly when mixture composition deviates from Lambda = 1.The injection control unit manages Lambda sensor heating in proportion to exhaust gas temperature.This avoids thermal shocks to the ceramic case due to contact with condensed water present in exhaust gas when the engine is cold.The measurement chamber and heater are built into a planar (laminated) ceramic element that offers the benefit of fast chamber heating. This allows closed loop (Lambda = 1) control within 10 seconds of engine start-up.1. Connecting element2. Protective sleeve3. Planar sensor element4. Ceramic support pipe5. Sensor seat6. Ceramic seal7. Protective pipeThe Lambda sensor works on the principle of an oxygen concentration chamber with solid electrolyte.The measurement chamber surfaces are coated with microporous layers of noble metals.1. Exhaust gas2. Reference air passage3. HeaterELECTRICAL SPECIFICATIONS:
- Heater power supply: 12 V
- Heater resistance: 0.5 - 1 kOhm.
CATALYTIC CONVERTER
At the same time, the three-way catalytic converter makes it possible to reduce the levels of the three pollutant gases present in exhaust gases:
- Unburnt hydrocarbons (HC);
- Carbon monoxide (CO);
- Nitrogen oxides (NOx).
Two types of chemical reaction take place inside the catalytic converter:
- Oxidation of CO and HC to carbon dioxide (CO2) and water (H2O)
- Reduction of NOx to nitrogen (N2).
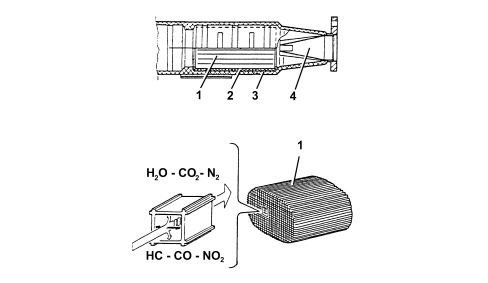
The converter consists of a structure, a metal gauze support to dampen impacts and vibrations, and a stainless steel outer casing that is resistant to high temperatures and atmospheric agents.The honeycomb structure is made from a ceramic material covered in an extremely thin layer of catalytically active substances, platinum or rhodium, which accelerate the chemical decomposition of the harmful substances contained in the exhaust gases which, when passing through the core cells at temperatures above 300 ° - 350°C, activate the catalyzers, setting off the oxidation/reduction reactions.A perforated steel cone improves the diffusion of the exhausts gases in the ceramic core cells to ensure the optimum efficiency and lifespan of the catalyzer.1. Ceramic structure2. Metal support3. Outer casing4. Perforated steel cone | The noble metals in the catalytic converter suffer chemical attack if lead is present, partly due to the high temperature inside the converter. For this reason, the use of leaded fuel should be avoided, otherwise the converter will be rapidly and irreversibly put out of service. Never use leaded fuel, not even for a short time or in an emergency. |